Course Details
Your Growth, Our Mission
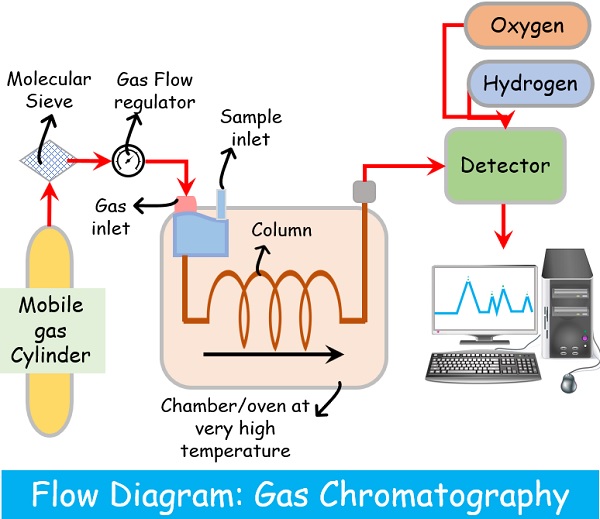
Course Description
Majority of modern instruments in the laboratory is computerized and provide incredible amounts of data. Methods that take advantage of the flood of data are now available by applying statistics which is the science of collecting, analyzing and interpreting data, facts can be established and a solid knowledge about the system and its processes can be obtained. This course designed to improve performance of lab staff for chemical testing with Gas Chromatography (GC) include method development and method validation to improvement this measurement methods of analysis.
The Training Course Will Highlight ?
Training Objective
Target Audience
The course is of interest for any person working in any analytical laboratory. Laboratory staff, Chemists, supervisors and technicians.
Training Methods
This interactive Training will be highly interactive, with opportunities to advance your opinions and ideas and will include;
- Lectures
- Workshop & Work Presentation
- Case Studies and Practical Exercise
- Videos and General Discussions
Daily Agenda
Day 1:
- Introduction
- History of Chromatography
- Symbols and terms of Chromatography
- Physical forces and interactions chromatography
- Fundamentals of chromatography
- Type of Chromatographic Analysis
- Gas Chromatography
- Objectives & Definitions
- Principals of GC
- Mobile and Stationary Phase
- Instrumentation of Gas Chromatography
- Mobile Phase
- Gas Supplies
- Sample Introduction, Injection Ports and Valves
- Columns
- Detectors
Day 2:
- GC operation
- Setup and GC Operation, Basic steps
- Sampling Techniques
- Programmed Temperature GC
- Peak Dispersion in a Chromatographic Column
- Your First Chromatogram
- From Chromatograms to Report
- Maintenance and troubleshooting
- Errors in Qualitative and Quantitative Analysis
- Correction of errors and improving accuracy
- Preventive maintenance
- Routine Maintenance
- Practical application for schedule maintenance
- How to replace spare parts
- Troubleshooting fixation
- Evaluation of GC
Day 3:
- Data Acquisition and Calibration
- Recorders & Data Acquisition
- Data analysis
- Data Acquisition and Processing
- Data Processing
- Calibration
- Data Acquisition and Processing System
- Calibration linked to GC performance
- Data Results
- Recorders & Data Acquisition
- Data analysis
- Data Acquisition and Processing
- Data Processing
- Application in analysis by Gas Chromatography
- Qualitative & Quantitation Analysis Strategies
- Performing Trace Analysis
- Errors in Qualitative and Quantitative Analysis
- Quality Control & Quality Assurance
- Statistical in analytical methods
- Data Validation of analysis results
- Quality Assurance Program
- Evaluation of analytical data
- Study Performance and Reporting
Day 4:
- Method development and validation
- Why is method validation necessary?
- How should methods be validated
- Method Validation process
- Method Calibration, and Standardization
- Certificate Reference Material (CRM), choice and preparation
- Correction of errors and improving Blank
- Instruments Calibration and Traceability
- Calibration Procedures, Certificate, and Documentation
- Standard calibration curve
- Standard addition calibration curve
- Internal standard calibration curve
- Linearity and Residual analysis in regression
- Measurement Uncertainly in testing and calibration
- Methods for uncertainty characterization
- Standard Deviation and Standard Error
- Measurement Uncertainty in chemical measurements
Day 5:
- Characteristic and steps of Method validation
- Selectivity
- Specificity
- Tolerance
- Linearity, Correlation Coefficient
- Accuracy & Precision
- Reliability (Repeatability, Reproducibility)
- Sensitivity, Detection Limit (LOD)
- Dynamic Range (LOQ, LOL, and LUR)
- Robustness
- The Evaluation of Results and Methods
- Errors in Analysis (Systematic, Random, and Gross)
- Correction of errors and improving accuracy
- Study Performance and Reporting
- Significant Figure & Rounding
- Reporting Analytical results and Archives
- Laboratory Certification
- Quality Audit (Internal and External)
- Practical exercises for Method Validation by Excel
Accreditation
BTS attendance certificate will be issued to all attendees completing minimum of 80% of the total course duration.
Quick Enquiry
Request Info
Related Courses
Your Growth, Our Mission

